Description
Supplement Facts
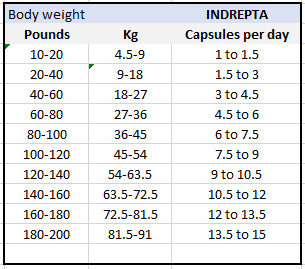
Average adult serving size 3 capsules taken 3 times per day
See chart below for more detailed suggestions
Capsules per container – 270
| INDREPTA C | DELIVER PER DAY (mg) |
| INGREDIENTS : | Per 9 capsules |
| Turmeric (Curcuma longa) 95% curcuminoids | 2000 |
| Trans-Resveratrol (Polygonum cuspidatum) 98% | 240 |
| Amentoflavone (Platycladus orientalis leaf) 20% | 150 |
| Quercetin dihydrate (Sophora japonica) 95% | 200 |
| Bioperine™ (Piper nigrim) 95% piperine | 10 |
| Silymarin (Silybum marianum) 80% | 80 |
| EGCG (Camellia sinensis) 98% polyphenols, 80% catechins, 50% EGCG | 135 |
| Hawthorne fruit (Crataegus pinnatifida bunge) 2% vitexins | 160 |
| Hawthorne leaf (Crataegus pinnatifida bunge) 2% vitexins | 160 |
| Forskolin (Coleus forskholii, 10% Forskolin) | 50 |
| Boswellic acid (Boswellia serrata) 90% | 500 |
| Escin (Aesculus hippocastanum) 20% | 150 |